Alternatively, continuous manufacturing, which is the preferred manufacturing process in automotive, food & beverage, and refining industries – has been slow to gain acceptance in pharmaceutical production, largely because of high startup costs.
Comparisons
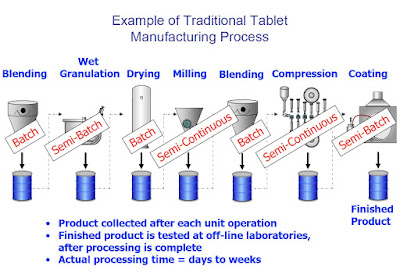
Batch Manufacturing:
All materials are charged before the start of processing and discharged at the end ofprocessing.
- Examples: Bin blending, lyophilization, some reactions
Continuous Manufacturing
Material is simultaneously charged and discharged from the process- Examples: Petroleum refining, much of food processing
VARIATIONS
Semi-Batch (Fed-batch)Manufacturing
Materials are added during processing and discharged at the end of processing.- Examples: Wet granulation, fermentation
Semi-Continuous Manufacturing
Like continuous manufacturing, but for a discrete time period.- Examples: Roller compaction, tablet compression
Although reliable, batch processing is viewed a slower manufacturing method for pharmaceuticals, and also less safe because of higher risk for contamination and errors between steps. Pharmaceutical manufacturers have no choice but to continually evaluate and implement the best possible production processes. Considering an estimated $50 billion per year is wasted on on inefficient processes in the pharmaceutical industry, it makes great sense to migrate toward continuous manufacturing.
Continuous manufacturing is faster, more efficient, and inherently safer. Improved safety is derived from rigid quality control requirements in continuous manufacturing. Considering this, the concern over large plant and equipment outlays looses is impact. Many experts maintain that continuous manufacturing is ultimately a far less costly production process (considering efficiency and safety), once the initial plant, equipment, and training costs are amortized.